RIDTS
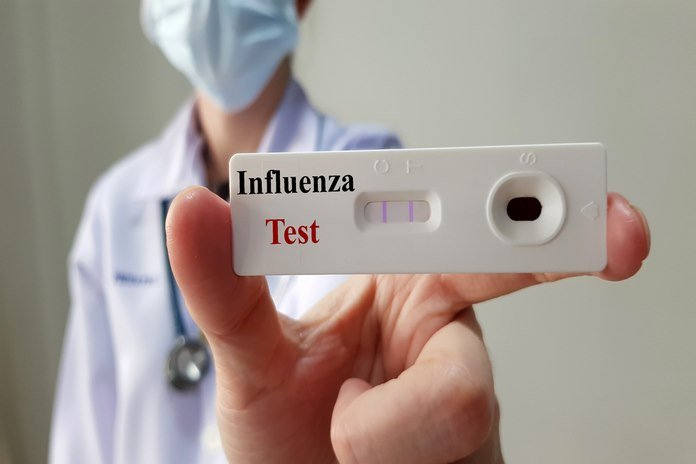
Rapid diagnostic assays for the detection of influenza antigens are commercially available and provide results in approximately 15 minutes with increased sensitivity and precision. The rapid diagnostic assays for influenza sold commercially do not require an outpatient setting and are exempt from CLIA regulations. The rapid diagnostic procedures vary according to the various influenza virus types. Some rapid influenza diagnostic tests necessitate an analyzer or reader to enhance sensitivity and standardize results. None of the analyses in the RIDTS assays provide evidence regarding the influenza virus A subtypes.
Different kinds of specimens, such as nasal swabs or washes and throat swabs, are required for the test to be valid. Specificity, notably sensitivity, of rapid influenza diagnostic tests are comparable to viral cultures and vary depending on the test. Due to the lower sensitivity of RIDTS, clinicians use other molecular assays, such as RT-PCR, to confirm the presence of the influenza virus, particularly during an influenza outbreak in the community. False-positive results are much less likely than false-negative results. As a consequence of interpreting a rapid influenza diagnostic test, physicians use the test’s estimated negative and positive values to determine the level of influenza activity in the community.
